Classes Of Organohalogens And Background Information
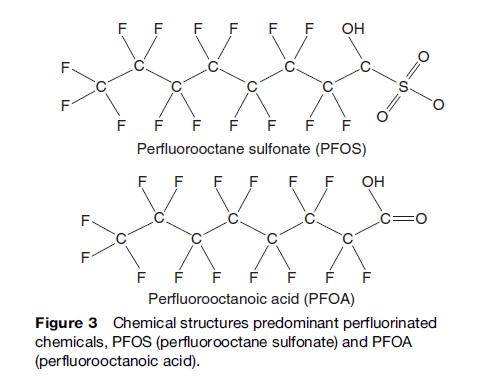
Chlorinated hydrocarbons were synthesized as early as 1830. Polychlorinated biphenyls (PCBs) were first synthesized in the early 1880s by Schmidt and Schultz (1881) and their commercial production began in 1929. Commercial PCB formulations were sold under a variety of trade names; for example, in the United States and Great Britain Aroclor was the most common trade name for PCBs. PCB mixtures were named according to their chlorine content. For instance, Aroclor 1254 contains 54% chlorine by weight, and Aroclor 1260 contains 60%. The PCB mixture formulations were different depending on the country of origin and were produced in Germany (Clofen), France (Phenoclor and Pyralene), Japan (Kanechlor), Italy (Fenclor), Russia (Sovol), and Czechoslovakia (Delor). PCB mixtures were produced for a variety of uses such as fluids in electrical transformers, capacitors, heat transfer fluids, hydraulic fluids, lubricating and cutting oils, and as additives in plastics, paints, copying paper, printing inks, adhesives, and sealants. Agricultural insecticide, DDT, was first synthesized before the turn of the 20th century by Zeilder in 1874. Its insecticidal value was discovered, and it was put to field use in the 1940s. Subsequently, this chlorine-containing insecticide replaced compounds with arsenic, lead, and copper, which were in use as insecticides. Application of DDT contributed to rapid reduction of malaria and other insect-borne diseases such as typhoid fever and cholera, and an astonishing associated increase in agricultural productivity in many regions of the world. The great success in the application of DDT during, and in the years following, the Second World War earned Paul Mu¨ ller a Nobel Prize in Medicine. The other organochlorines, HCHs and CHLs, were introduced in 1945 and these insecticides also contributed to human welfare as agricultural and domestic pest control agents. Owing to persistent, bioaccumulative, toxic properties and long-term health effects, organochlorine compounds were banned or severely restricted in the early 1970s in several developed countries (Loganathan and Kannan, 1994). Following this initial restrictive action, persistent, bioaccumulative, and toxic chemicals (PBTs) were the subject of a concerted regional, national, and international effort to limit the production, use, and control the disposal of materials no longer in use. The next class of organohalogens of concern is brominated compounds, including polybrominated diphenyl ethers (PBDEs) and polybrominated dibenzo-p-dixions/ furans (PBDDs/PBDFs). PBDEs constitute an important group of flame retardants. PBDEs are added to consumer products so the products will not catch fire or will burn more slowly if exposed to flame or heat. PBDEs are added to plastics, upholstery, fabrics, and foams and in common products such as computers, television sets, mobile phones, furniture, and carpet pads. Nearly 90% of electrical and electronic appliances contain PBDEs, which are added as flame retardants that afford upto 15 times greater escape time in case of a fire. PBDDs/PBDFs are relatively less toxic than chlorinated dioxins and are formed during heating or incineration of polybrominated biphenyls (PBBs) and PBDEs. Low levels of PBDDs/PBDFs detected in environmental samples suggest relatively lower exposure to biota (fish) and humans to these compounds. In contrast to PCBs, PBDEs are currently being produced and used in household materials. PBDEs are primarily indoor pollutants. PBDEs leach into the environment when household wastes decompose in landfills or are incompletely incinerated. Human health concerns stem from the fact that PBDEs are persistent, bioaccumulative, and structurally similar to PCBs (Figure 2). PBDE concentrations are rapidly increasing in the global environment and in human blood, breast milk, liver, etc. Although these chemicals are ubiquitous in the environment and bioaccumulate in wildlife and humans, little is known about their potential toxic properties (Kodavanti, 2005).Perfluorinated compounds are another group of organic compounds in the persistent organohalogen family. PFCs are used in a variety of specialized consumer and industrial products (Senthil Kumar, 2005). PFCs are used in metal-plating baths, surfactants, cleaning products, rust inhibitors, fire-fighting applications, starting materials for polymers, herbicide and insecticide formulations, cosmetics, shampoos, pharmaceuticals, water and oil repellent coatings for fabrics and paper, greases and lubricants, paints, polishes, upholstery, textiles, carpets, soil/stainresistance coatings, mining and oil well surfactants, acid mist suppressants, electronic etching baths, alkaline cleaners, floor polishes, photographic film, and denture cleaners and adhesives. PFCs are also used in paper protection, including food contact applications (plates, food containers, bags, and wraps) and non-food-contact applications (folding cartons, masking papers; Kannan et al., 2004). The chemical stability and nondegradable nature of PFCs, coupled with their widespread use, has led to global environmental contamination and accumulation of PFCs in aquatic and terrestrial organisms, including humans. Perfluorooctane sulfonate (PFOS, Figure 3) and perfluorooctanoic acid (PFOA, Figure 3) have been routinely detected in environmental matrices, wildlife, and human tissues (Kannan et al., 2004). Detectable amounts of PFOS were also found in human blood samples obtained from individuals residing in a number of countries. These findings have raised concerns about environmental contamination by perfluorinated compounds and their possible impacts on ecosystems and on human health. Although perfluorinated chemicals are ubiquitous in the global environment and bioaccumulate in wildlife and humans, their toxic properties are still under investigation.
Chlorinated Compounds
Physicochemical Properties
Human-made organochlorine compounds possess unique properties that render them highly persistent in the global environment, causing chronic toxicity to wildlife and humans. PCBs are colorless to light yellow, have no smell, and are tasteless oily liquids or solids. Some PCBs are volatile and may exist as a vapor in air. The physicochemical properties of PCBs vary widely and depend on the number and positions of chlorine atoms in the biphenyl rings. PCBs resist both acids and alkalis and have thermal stability. This has made them useful in a wide variety of industrial applications including dielectric fluids in transformers and capacitors, heat transfer fluids, and lubricants. Generally, PCBs are relatively insoluble in water and the solubility decreases with increased chlorination. PCBs are readily soluble in nonpolar organic solvents and biological lipids. When PCBs are burned at high temperatures, the products of combustion include polychlorinated dibenzofuran (PCDFs) and polychlorinated dibenzo-p-dioxins (PCDDs), which are more hazardous than PCBs itself. DDT compounds, HCH isomers, and chlordane compounds have properties similar to certain higher-chlorinated and lower-chlorinated PCBs, respectively. The physical and chemical stability of the organochlorines contributes to their persistence in the environment and is responsible for the environmental and health problems caused by these compounds.
Our Advantages
- Quality Work
- Unlimited Revisions
- Affordable Pricing
- 24/7 Support
- Fast Delivery
Order Now