Introduction
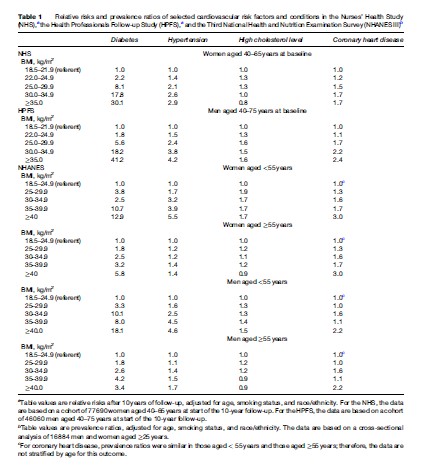
The prevalence of obesity has increased sharply during the past few decades, and this condition is now recognized as a major contributor to the global burden of disease. Adiposity, or excess body fat, is often defined in terms of body mass index (BMI), which is calculated by dividing weight in kilograms (kg) by the square of height in meters (m). For adults, the World Health Organization (WHO) defines underweight as BMI <18.5, normal weight as BMI 18.5 to <25, overweight as BMI 25 to <30, and obesity as BMI 30 kg/m2. Using this standard, about half of European adults are overweight or obese, and urban areas of many developing countries have a similar prevalence. In the United States, two of every three adults are either overweight or obese. According to the U.S. National Health and Nutrition Examination Surveys (NHANES), although the percentage of adults in the overweight but not obese category has remained relatively stable for the past 30 years, there has been a doubling in obesity prevalence – from 14.5 to 32.2% – between 1980 and 2004. For children there are no definitions of overweight and obesity that are accepted worldwide. NHANES data indicate that the prevalence of childhood obesity in the United States, defined as a BMI 95th percentile for children of the same age and sex according to the 2000 Centers for Disease Control and Prevention Growth Charts, increased from approximately 6 to 16% between 1980 and 2004. Worldwide the prevalence of overweight and obesity has been increasing in both industrialized countries and urban areas of developing countries. WHO estimates that 400 million adults worldwide were obese in 2005; that number is projected to jump to 700 million by 2015. Although viewed more as a cosmetic than a health problem by the general public and some health-care professionals, excess weight is a major risk factor for chronic disease and other medical complications. Epidemiologic research has quantified the impact of overweight and obesity on premature mortality, cardiovascular disease (CVD), type 2 diabetes, osteoarthritis, gallbladder disease, some types of cancer, and other adverse outcomes (Figure 1). Direct dose–response relationships between increasing BMI and lifetime risks of various conditions have been observed in nationally representative samples, such as NHANES, and in large cohorts followed for lengthy periods, such as the Nurses’ Health Study, the Health Professionals Follow-up Study, and the Framingham Heart Study (Table 1). One conservative estimate derived from five long-term prospective cohort studies is that obesity accounts for >280 000 deaths each year in the United States. The direct medical costs of obesity have been estimated at 5.5 to 7% of U.S. total health-care expenditures. The substantial morbidity and mortality associated with overweight and obesity point to the pressing need to educate the public and medical communities about the hazards of excess weight and to remove the barriers to healthy eating and greater physical activity.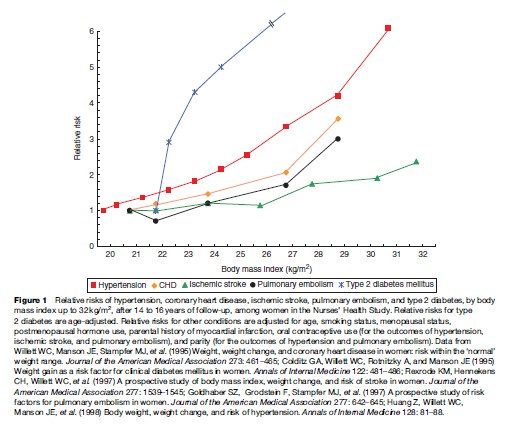
Mortality
Most epidemiologic studies report a Uor J-shaped relationship between BMI and mortality, with death rates elevated in persons with very low and high relative body weights. While extremes of adiposity or of leanness are clearly deleterious, defining the precise range of weights associated with minimal mortality or optimal health has been controversial because of methodologic limitations that can distort inferences about the role of weight in health outcomes. One such problem is the failure to consider the effects of preexisting overt or occult disease. Illness-induced weight loss may obfuscate positive dose–response relationships between adiposity and mortality. To reduce this reverse causation bias, investigators typically limit analyses to participants who appear healthy at baseline, and they sometimes exclude deaths occurring during the first few years of follow-up based on the assumption that many of these deaths are due to preexisting disease that may have already influenced weight. Reverse causation is an especially strong threat to the validity of (1) studies of the elderly, because older people are more likely to have chronic diseases that lead to weight loss, and (2) studies with short follow-up periods, because certain conditions (e.g., preclinical cancers and cardiorespiratory diseases) can cause insidious weight loss beginning years before diagnosis. Another limitation is insufficient control for cigarette smoking. Because smoking is more prevalent among leaner individuals and is also a strong independent risk factor for death, not adjusting for its effect will produce an artifactually elevated mortality in lean subjects. Although such confounding can be partially controlled by statistical techniques, the best estimates are likely to come from studies of never smokers. A third issue is that of inappropriate statistical control for biologic consequences of obesity, such as hypertension, dyslipidemia, or hyperglycemia, which eliminates physiologic pathways by which obesity affects mortality and morbidity, thus attenuating observed relationships. Early studies did not fully address these issues. A seminal investigation that did attempt to control for confounders was the American Cancer Society’s Cancer Prevention Study I, a cohort of 750 000 U.S. adults followed from 1960 to 1972. Although the analysis did not consider the potential for reverse causation bias, it did stratify the data by smoking status. Among never smokers, mortality was minimal at or somewhat below the cohort’s average relative weight. The excess mortality observed in the lowest weight groups was more pronounced among current or ex-smokers than among never smokers and was mainly due to cancers of the lung, bladder, and pancreas, strongly implicating smoking. A reanalysis of these data using BMI instead of relative weights and excluding those with a history of smoking, cancer, CVD, or recent weight loss showed an even clearer pattern of increasing mortality with increasing weight (Stevens et al., 1998), as did an analysis of a second American Cancer Society cohort of >1 million adults followed from 1982 to 1996 (Calle et al., 1999) (Figure 2(a)). Among healthy never smokers with stable weight, the lowest mortality occurred at BMI 23.5 to 24.9 for men and 22.0 to 23.4 for women. Similar results have been observed in other large cohorts. For example, analyses of Nurses’ Health Study data from 1976 to 1992 showed that the gradient between BMI and mortality became markedly stronger when biases due to smoking and reverse causation were simultaneously controlled (Figure 2(b)). Data from a recent 10-year study of 527 000 U.S. adults aged 50 to 71 followed from 1995 to 2005 are also illustrative. In analyses that were limited to never smokers to eliminate confounding by smoking status and that also used BMI at age 50 rather than BMI at study baseline as the exposure variable to reduce the likelihood of reverse causation, the observed relationship between excess weight and mortality was notably strengthened; there was an increased risk of death of 20 to 40% and of 100 to 200% among overweight and obese persons, respectively (Adams et al., 2006) (Figure 2(c)). Taken together the results of these and other methodologically rigorous studies support WHO guidelines that set the healthy BMI range for adults at 18.5 to <25.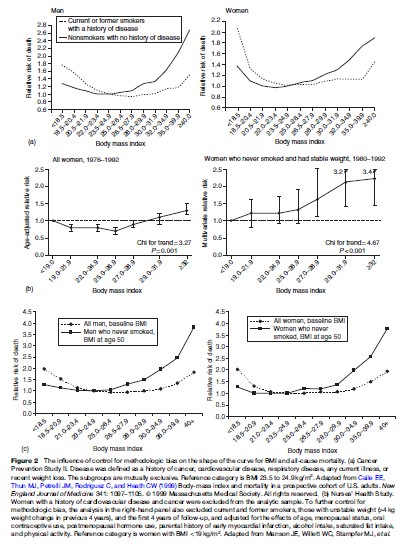 Weight in childhood has been less extensively studied than weight in adulthood as a predictor of health outcomes. However, an investigation of 227 000 Norwegian adolescents aged 14 to 19 who were followed for 32 years found that a high BMI predicted premature mortality (Engeland et al., 2003). Compared with those with baseline BMI in the 25th to 75th percentile of a U.S. reference population, females with baseline BMI in the 85th to 95th percentile and those with BMI > 95th percentile were 30% and 100% more likely to die in early to middle adulthood, respectively. The corresponding figures for males were 30% and 80%.
Most estimates of the obesity–mortality association are derived from studies of white populations of European ancestry. Although additional studies in diverse groups are needed, available data do not indicate that the biological relation between adiposity and mortality varies importantly across racial/ethnic lines. However, to the extent that there are racial/ethnic differences in the reliability of BMI as a measure of adiposity, studies that utilize more direct measures of adiposity (e.g., percentage body fat) will be useful for refining estimates of the mortality impact of overweight and obesity in various populations. Nevertheless, there is currently no compelling evidence to support the exclusion of any racial/ethnic group from the WHO definitions of overweight and obesity.
It should be emphasized that studies of total mortality are inherently insensitive in pinpointing the weight range associated with optimal health. There are two reasons for this insensitivity. First, obesity, similar to almost every other risk indicator, is unlikely to influence all specific causes of death. In large investigations of multiple outcomes (e.g., the Nurses’ Health Study), the relative risks associated with high BMI are far higher for the incidence of specific diseases, including type 2 diabetes, myocardial infarction, and ischemic stroke, than for all-cause mortality. Similarly, many studies show that intentional weight loss reduces various disease-specific risks, while only limited data support the notion that such weight loss reduces total mortality. One study in the latter category is the Swedish Obese Subjects Study, a large nonrandomized trial of health outcomes among bariatric surgery patients versus matched obese control subjects, which recently found that, over an 11-year period, total mortality was significantly lower in the surgery group, who maintained an average loss of 14 to 25% of body weight (depending on surgery type), than in the control group, whose weight remained stable (Sjostrm et al., 2007). The second reason that use of mortality outcomes may not be the best way to determine optimal weights is that mortality is only a small part of the substantial burden of disease caused by conditions such as angina pectoris, degenerative arthritis, diabetes, hypertension, and nonfatal CVD.
Weight in childhood has been less extensively studied than weight in adulthood as a predictor of health outcomes. However, an investigation of 227 000 Norwegian adolescents aged 14 to 19 who were followed for 32 years found that a high BMI predicted premature mortality (Engeland et al., 2003). Compared with those with baseline BMI in the 25th to 75th percentile of a U.S. reference population, females with baseline BMI in the 85th to 95th percentile and those with BMI > 95th percentile were 30% and 100% more likely to die in early to middle adulthood, respectively. The corresponding figures for males were 30% and 80%.
Most estimates of the obesity–mortality association are derived from studies of white populations of European ancestry. Although additional studies in diverse groups are needed, available data do not indicate that the biological relation between adiposity and mortality varies importantly across racial/ethnic lines. However, to the extent that there are racial/ethnic differences in the reliability of BMI as a measure of adiposity, studies that utilize more direct measures of adiposity (e.g., percentage body fat) will be useful for refining estimates of the mortality impact of overweight and obesity in various populations. Nevertheless, there is currently no compelling evidence to support the exclusion of any racial/ethnic group from the WHO definitions of overweight and obesity.
It should be emphasized that studies of total mortality are inherently insensitive in pinpointing the weight range associated with optimal health. There are two reasons for this insensitivity. First, obesity, similar to almost every other risk indicator, is unlikely to influence all specific causes of death. In large investigations of multiple outcomes (e.g., the Nurses’ Health Study), the relative risks associated with high BMI are far higher for the incidence of specific diseases, including type 2 diabetes, myocardial infarction, and ischemic stroke, than for all-cause mortality. Similarly, many studies show that intentional weight loss reduces various disease-specific risks, while only limited data support the notion that such weight loss reduces total mortality. One study in the latter category is the Swedish Obese Subjects Study, a large nonrandomized trial of health outcomes among bariatric surgery patients versus matched obese control subjects, which recently found that, over an 11-year period, total mortality was significantly lower in the surgery group, who maintained an average loss of 14 to 25% of body weight (depending on surgery type), than in the control group, whose weight remained stable (Sjostrm et al., 2007). The second reason that use of mortality outcomes may not be the best way to determine optimal weights is that mortality is only a small part of the substantial burden of disease caused by conditions such as angina pectoris, degenerative arthritis, diabetes, hypertension, and nonfatal CVD.
Cardiovascular Disease
Obesity is associated with an increased risk of coronary heart disease (CHD), stroke, venous thromboembolism (VTE), heart failure, and cardiomyopathy. Obesity raises these risks partly through its effects on established coronary risk factors such as hypertension, dyslipidemia, and insulin resistance. Excess weight is also associated with several novel risk factors for CVD and diabetes, including elevations in thrombotic markers, such as fibrinogen and plasminogen activator inhibitor-1, and in inflammatory markers, such as interleukin-6 and C-reactive protein. Independent of its effect on these established and novel risk factors, obesity also appears to have a residual impact on risk of CVD itself, a finding most pronounced in long-term prospective studies. Consistent with the excess CHD risk observed even among persons at the upper limit of the healthy weight range, the prevalence and incidence of some cardiovascular risk factors rises rapidly at BMI > 20. In the Nurses’ Health Study, for example, the relative risks of incident hypertension were 1.00 for BMI < 20 (the referent category), 1.15 for BMI 20 to 20.9, 1.35 for BMI 21 to 21.9, 1.56 for BMI 22 to 22.9, 1.80 for BMI 23 to 23.9, and 2.12 for BMI 24 to 24.9. Relative risks continued to climb with increasing degrees of overweight, reaching 6.12 for BMI≥ 31. In a study of 14 000 healthy female employees of the British company Marks and Spencer, marked age-adjusted increases in systolic and diastolic blood pressure, serum total cholesterol, low-density lipoprotein cholesterol, triglycerides, and fasting blood glucose, as well as significant decreases in high-density lipoprotein cholesterol, were observed across seven BMI categories ranging from <20 to >30 (Ashton et al., 2001). The Framingham Heart Study has also demonstrated that many men and women with BMI 23 to 25 have abnormalities in serum lipids, glucose tolerance, and blood pressure compared with those with BMI <23, and that most individuals with BMI >25 have such abnormalities. Although most investigations of weight and metabolic risk factors have been conducted in Western populations, a study of 1610 rural Chinese peasants also found a striking monotonic rise in adverse lipid, glucose, and blood pressure profiles as BMI increased from <18 to >24 (Hu et al., 2000). Strong associations between relative body weight and cardiovascular risk factors have also been observed in children and adolescents. In the Bogalusa Heart Study, which examined 9157 Louisiana children aged 5 to 17, lipid, insulin, and blood pressure levels did not vary with BMI at levels below the 85th percentile of a national reference population (Freedman et al., 1999). However, the probability of having an adverse lipid, blood pressure, or insulin profile was substantially higher in children with BMI above the 95th percentile than in children with BMI below the 85th percentile. Overweight children were 2.4, 3, 3.4, 7.1, and 12.6 times more likely than normal-weight children to have a total cholesterol level > 200 mg/dL, low-density lipoprotein cholesterol > 130 mg/dL, high-density lipoprotein cholesterol < 35 mg/dL, triglycerides≥ 130 mg/dL, and fasting insulin 95th percentile, respectively. Overweight children were also 4.5 times more likely to have an elevated systolic blood pressure and 2.4 times more likely to have an elevated diastolic blood pressure than their normal-weight counterparts. Although few data are available regarding the long-term benefits of intentional weight loss on CVD incidence, a number of mostly small clinical trials of various designs demonstrate benefits of weight loss among overweight and obese individuals on specific cardiovascular risk factors, including blood pressure, glucose tolerance, and lipoprotein profile. The Swedish Obese Subjects Study found that, over a 10-year period, ‘rates of recovery’ from hypertension, dyslipidemia, and diabetes were significantly higher in the bariatric surgery group than in the control group (Sjostrom et al., 2004).Coronary Heart Disease
Observational studies, including many long-term prospective cohort studies, have demonstrated a strong association between excess weight and CHD. The relationship appears to be linear, and even individuals of average weight at midlife are at increased risk compared with their leaner counterparts. For example, in the Nurses’ Health Study, even after adjustment for the effects of age, smoking, menopausal status, menopausal hormone use, and parental history of myocardial infarction, the relative risk of CHD was 1.19 for women with BMI 21 to 22.9, 1.46 for BMI 23 to 24.9, 2.06 for BMI 25 to 28.9, and 3.56 for BMI ≥ 29, compared with women with BMI <21. Nearly two-fifths (37%) of CHD incidence in this cohort was attributable to excess weight, defined as BMI ≥21. Weight gain between age 18 and midlife was also associated in a dose-dependent fashion with increased CHD risk. Compared with women with stable weight women who gained 5 to 7.9 kg were 1.25 times more likely to develop CHD, and those who gained 20 kg were 2.65 times more likely to do so. Viewed alternatively, CHD risk increased by 3.1% for each kg gained. More than one quarter (27%) of the overall incidence in this cohort could be accounted for by weight gains of 5 kg or more. In the British Regional Heart Study, a 15-year follow-up of nearly 8000 middle-aged men, the risk of major coronary events and cardiovascular mortality increased progressively with increasing BMI, even after controlling for age, smoking, social class, alcohol consumption, and physical activity (Shaper et al., 1997). An increase in body weight equivalent to 1 BMI unit from 20.0 onward was predictive of a 10% increase in the rate of coronary events. In a cohort of 16 000 middle-aged Finnish men and women followed for 15 years, each 1-unit increase in BMI was associated with a 4 to 5% increase in CHD mortality ( Jousilahti et al., 1996). A 26-year follow-up of 5200 participants in the Framingham Heart Study found that high relative weight at baseline was directly associated with CHD, coronary mortality, and heart failure independent of age, cholesterol, systolic blood pressure, smoking, and other cardiovascular risk factors. In agreement with the Nurses’ Health Study findings, the proportion of CHD attributable to excess weight was estimated to be roughly 25%, and weight gain during young and middle adulthood was associated with increased risk of CVD in both genders. In addition to the amount of excess fat, the regional distribution of such fat also affects cardiovascular health. Adipose tissue in the waist, abdomen, and upper body is more metabolically active than that in the hip, thigh, or buttocks, and abdominal fat accumulation is an important predictor of dyslipidemia, hypertension, and CHD as well as of type 2 diabetes. The heightened sensitivity of abdominal fat cells to lipolytic agents and the subsequent direct delivery of free fatty acids and glycerol to the liver, thus inducing insulin resistance, are possible pathophysiologic explanations for these observed associations. Abdominal adiposity is often estimated using waist circumference or waist-to-hip ratio. A waist-to-hip ratio higher than 0.80 in women and 0.95 in men – or a waist circumference of 35 inches in women and 40 inches in men – is associated with increased cardiovascular risk.Stroke
The clear weight-related increases in blood pressure, lipids, and blood glucose described earlier should be expected to translate into an elevated risk of stroke among overweight and obese individuals. However, data regarding this relationship are less consistent than they are for CHD. Some of the inconsistency may be due to the fact that, until recently, stroke subtypes have rarely been examined. Several recent prospective studies that have modeled ischemic and hemorrhagic stroke as distinct endpoints have found that excess weight is a stronger risk factor for the former than for the latter outcome. In perhaps the largest investigation of stroke in relation to obesity to date, a 14-year follow-up of 235 000 middle-aged male civil servants in Korea, each 1-unit increase in BMI was associated with a clearly significant 6% increase in risk of ischemic stroke and a marginally significant 2% increase in risk of hemorrhagic stroke, after adjustment for age, smoking, alcohol consumption, exercise habit, and salary level (Song et al., 2004). In the Nurses’ Health Study, BMI and weight gain were associated with an increased risk of ischemic stroke, but not hemorrhagic stroke, over 16 years. After factoring out the effects of age, smoking, menopausal status, and menopausal hormone use, the incidence of ischemic stroke increased steadily with degree of overweight; compared with women with BMI <21, relative risks for women with BMIs of 27 to 28.9, 29 to 31.9, and ≥32 were 1.75, 1.90, and 2.37, respectively. Conversely, there was an inverse (albeit nonsignificant) relationship between excess weight and hemorrhagic stroke, with the highest risk among women in the leanest BMI category. A strikingly similar pattern of findings was observed in the Women’s Health Study, in which 39 000 U.S. female health professionals were followed for 10 years (Kurth et al., 2005). One possible explanation for the inverse relation between BMI and hemorrhagic stroke is that low serum cholesterol, which is more prevalent among the lean, may be associated with increased blood vessel fragility or altered endothelial function. In contrast to these results, the Physicians’ Health Study, a 12-year follow-up of 21 000 U.S. male physicians, found that high BMI predicted a significantly elevated risk of hemorrhagic as well as ischemic stroke (Kurth et al., 2002). A comparison of men with BMI ≥ 30 with those with BMI <23 indicated an approximate doubling of risk for both stroke subtypes. Additional research is needed to clarify the role of obesity in hemorrhagic stroke.Venous Thromboembolism
Studies of risk factors for VTE, conducted mainly in hospitalized patients, have generally demonstrated an association between excess weight and deep-vein thrombosis or pulmonary embolism. Two large prospective studies of community-dwelling populations support this association. Among 113 000 women in the Nurses’ Health Study, the risk of developing primary pulmonary embolism over 14 years of follow-up was nearly three times higher among those with BMI≥29 as compared with those with BMI <21. In an 8-year follow-up of 19 000 U.S. adults aged≥45 in the Atherosclerosis Risk in Communities Study and the Cardiovascular Health Study, individuals with BMI ≥ 30 were 70% more likely to develop VTE than were those with BMI <30 (Tsai et al., 2002).Type 2 Diabetes
Excess weight plays a critical role in the etiology of type 2 diabetes, which is the main cause of kidney failure, limb amputations, and new-onset blindness in U.S. adults, and a major risk factor for premature CHD and stroke. Obesity, especially abdominal obesity, causes insulin resistance and compensatory hyperinsulinemia, which in turn are implicated in the development of type 2 diabetes. The marked rise in obesity among U.S. adults between 1991 and 2001 has been accompanied by a 61% increase in diabetes prevalence. Data from the nationally representative NHANES III show a direct relationship between BMI and prevalence of type 2 diabetes in U.S. adults (Figure 3). Strong associations between BMI and type 2 diabetes have also been consistently observed in ethnically diverse populations, including Europeans, Japanese, Mexicans, and Native Americans. Of five ‘lifestyle’ variables – obesity, lack of physical activity, poor diet, current smoking, and alcohol abstinence – examined in the Nurses’ Health Study, excess body weight was by far the most important predictor of diabetes onset among 85 000 women. The risk of developing type 2 diabetes over 16 years of follow-up was nearly 40-fold higher for women with BMI ≥ 35 and20-fold higher for women with BMI 30 to 34.9, as compared with women with BMI 23. Even a BMI falling within the high end of the normal range (23 to 24.9) was associated with a nearly threefold increase in risk over that experienced by women with BMI ≥23. Taking women with BMI <25 as the referent, the 10-year relative risk of incident diabetes was 4.6 among women with BMI 25 to 29.9, 10 among those with BMI 30 to 34.9, and 17 among those with BMI 35. In the Health Professionals Follow-up Study, taking men with BMI <25 as the referent, the 10-year relative risk was 3.5 for those with BMI 25 to 29.9, 11.2 for those with BMI 30 to 34.9, and 23.4 for those with BMI ≥ 35 (Field et al., 2001). In the NHANES Epidemiologic Follow-up Study, a cohort of 8545 adults aged 18 followed for 9 years, diabetes incidence climbed rapidly as BMIs increased above 22 (Ford et al., 1997). For each 1-unit increase in BMI (about 2.7 to 3.6 kg for the average participant), the risk of developing diabetes increased 12%. The estimated proportion of diabetes in U.S. adults attributable to excess weight (BMI≥22) was approximately 70% after factoring out the effects of smoking, alcohol use, cholesterol, blood pressure, and antihypertensive medication use.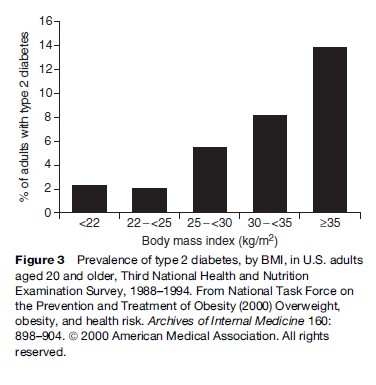 In these studies, weight gain during adulthood was also associated with a significant increase in risk of developing type 2 diabetes, although the magnitude of the estimates varied. In the NHANES Epidemiologic Follow-up Study individuals who gained 5 to 7.9 kg over the preceding 10 years experienced a doubling of risk over a 9-year period as compared with their stable-weight counterparts, and those who gained 20 kg had a fourfold risk increase. In the Nurses’ Health Study, when compared with women with stable weight, women who gained 5 to 7.9 kg from early to mid-adulthood also had a near doubling of risk over a 14-year follow-up, but women who gained 20 kg had more than a 12-fold risk increase.
Given the widespread prevalence of diabetes, even modest risk reductions could have large effects on the global burden of this disease. Although metabolic benefits of weight loss have not been consistently observed in community-based settings, intervention studies in high-risk populations suggest that intentional weight loss, either alone or combined with physical activity, improves glucose levels and insulin action among persons with type 2 diabetes and lowers diabetes risk among overweight individuals. In the 10-year Swedish Obese Subjects Study, bariatric surgery patients without diabetes at baseline were 75% less likely to develop the condition than were matched obese controls, while bariatric surgery patients with diabetes were 3.5 times more likely to ‘recover’ from the condition than controls (Sjostrom et al., 2004). In the Da Qing Impaired Glucose Tolerance and Diabetes Study, 577 middle-aged Chinese men and women with impaired glucose tolerance were randomized to one of three treatment groups – diet only, exercise only, or diet plus exercise – or to a control group (Pan et al., 1997). Over 6 years the three interventions were associated with statistically significant reductions of 31%, 46%, and 42% in diabetes risk, respectively. In the Finnish Diabetes Prevention Study, 522 middle-aged overweight men and women with impaired glucose tolerance were randomly assigned to either an intensive lifestyle intervention designed to promote healthy eating and exercise patterns or a control group (Tuomilehto et al., 2001). Persons in the diet and exercise intervention group lost significantly more weight than did those in the control group (3.5 vs. 0.8 kg) and reduced their risk of developing diabetes by 58% over a 3-year interval. The U.S. Diabetes Prevention Program, a 3-year randomized trial that enrolled 3234 men and women aged 25 to 85 with impaired glucose tolerance, also reported a 58% reduction in diabetes risk among the intervention group, whose members, on average, exercised 30 minutes per day and lost 5 to 7% of their body weight during the trial (Diabetes Prevention Program Research Group, 2002). This trial oversampled older individuals as well as members of ethnic groups who suffer disproportionately from diabetes (i.e., African-, Hispanic-, and Asian-Americans; Pacific Islanders; and American Indians) and found that the lifestyle intervention was effective in reducing diabetes risk in all age and ethnic groups. An ongoing large randomized trial, the Look AHEAD (Action For Health in Diabetes) trial, should provide valuable information about the long-term ( 10 years) effects of sustained weight loss through decreased caloric intake and exercise on the risk of CVD in obese individuals with diabetes; results are expected in 2012.
In these studies, weight gain during adulthood was also associated with a significant increase in risk of developing type 2 diabetes, although the magnitude of the estimates varied. In the NHANES Epidemiologic Follow-up Study individuals who gained 5 to 7.9 kg over the preceding 10 years experienced a doubling of risk over a 9-year period as compared with their stable-weight counterparts, and those who gained 20 kg had a fourfold risk increase. In the Nurses’ Health Study, when compared with women with stable weight, women who gained 5 to 7.9 kg from early to mid-adulthood also had a near doubling of risk over a 14-year follow-up, but women who gained 20 kg had more than a 12-fold risk increase.
Given the widespread prevalence of diabetes, even modest risk reductions could have large effects on the global burden of this disease. Although metabolic benefits of weight loss have not been consistently observed in community-based settings, intervention studies in high-risk populations suggest that intentional weight loss, either alone or combined with physical activity, improves glucose levels and insulin action among persons with type 2 diabetes and lowers diabetes risk among overweight individuals. In the 10-year Swedish Obese Subjects Study, bariatric surgery patients without diabetes at baseline were 75% less likely to develop the condition than were matched obese controls, while bariatric surgery patients with diabetes were 3.5 times more likely to ‘recover’ from the condition than controls (Sjostrom et al., 2004). In the Da Qing Impaired Glucose Tolerance and Diabetes Study, 577 middle-aged Chinese men and women with impaired glucose tolerance were randomized to one of three treatment groups – diet only, exercise only, or diet plus exercise – or to a control group (Pan et al., 1997). Over 6 years the three interventions were associated with statistically significant reductions of 31%, 46%, and 42% in diabetes risk, respectively. In the Finnish Diabetes Prevention Study, 522 middle-aged overweight men and women with impaired glucose tolerance were randomly assigned to either an intensive lifestyle intervention designed to promote healthy eating and exercise patterns or a control group (Tuomilehto et al., 2001). Persons in the diet and exercise intervention group lost significantly more weight than did those in the control group (3.5 vs. 0.8 kg) and reduced their risk of developing diabetes by 58% over a 3-year interval. The U.S. Diabetes Prevention Program, a 3-year randomized trial that enrolled 3234 men and women aged 25 to 85 with impaired glucose tolerance, also reported a 58% reduction in diabetes risk among the intervention group, whose members, on average, exercised 30 minutes per day and lost 5 to 7% of their body weight during the trial (Diabetes Prevention Program Research Group, 2002). This trial oversampled older individuals as well as members of ethnic groups who suffer disproportionately from diabetes (i.e., African-, Hispanic-, and Asian-Americans; Pacific Islanders; and American Indians) and found that the lifestyle intervention was effective in reducing diabetes risk in all age and ethnic groups. An ongoing large randomized trial, the Look AHEAD (Action For Health in Diabetes) trial, should provide valuable information about the long-term ( 10 years) effects of sustained weight loss through decreased caloric intake and exercise on the risk of CVD in obese individuals with diabetes; results are expected in 2012.
Cancer
In the nearly three decades since the first Cancer Prevention Study of 750 000 U.S. adults reported a highly significant age and smoking-adjusted 33% increased risk of cancer death among obese men and a 55% increase among obese women, hundreds of investigations have examined the association between weight and cancer in greater detail. To date the evidence relating BMI to cancer incidence is strongest for endometrial, postmenopausal breast, kidney, gallbladder, colon, and esophageal cancer; recent data also suggest a link between obesity and pancreatic, liver, and stomach cancer. However, the consistency of some site-specific findings varies by gender; the evidence linking obesity to colon cancer is more compelling for men than for women, and the converse is true for gallbladder cancer. Also, obesity has not been strongly implicated in the development of some of the most common cancers, including those of the lung and prostate. Moreover, premenopausal breast cancer appears to occur slightly less frequently among overweight women, possibly because of the greater prevalence of menstrual irregularities and anovulation at higher weights. Alternatively, this finding may simply be an artifact of earlier tumor detection among leaner women. Not only do obese women have lower participation rates in cervical and breast cancer screening programs, but the presence of excess fat complicates tumor detection when obese women do undergo clinical examination. Thus, heavier patients may have more advanced disease at time of diagnosis and consequently benefit less from adjuvant chemotherapy. Indeed, in contrast to the breast cancer incidence findings, an inverse association between obesity and breast cancer mortality is not seen among premenopausal women. Data from the Cancer Prevention Study II, a 16-year follow-up of 900 000 U.S. adults, suggest that 15 to 20% of all U.S. cancer deaths can be attributed to overweight and obesity (Calle et al., 2003). Table 2 provides estimated relative risks and percentages of site-specific cancers attributable to excess weight in the United States and the European Union. Among U.S. adults aged 50 to 69, excess weight accounts for more than one-half of endometrial and esophageal cancers; more than one third of kidney, gallbladder, stomach, and male colorectal cancers; more than one-fourth of pancreatic cancers; and more than one-fifth of female colorectal and postmenopausal breast cancers.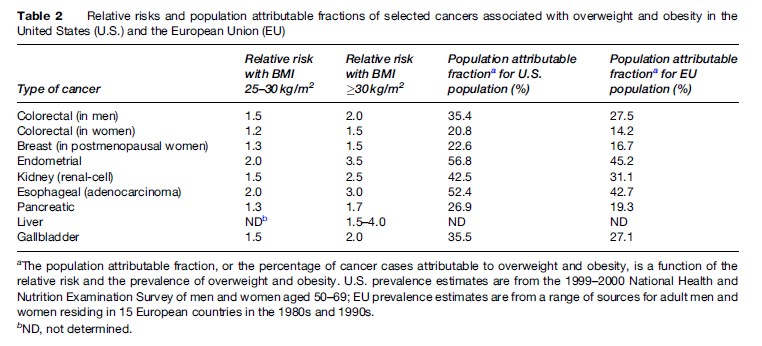 The biologic mechanisms by which obesity may raise cancer risk are not entirely clear and may vary by cancer site. It may be that obesity-associated insulin resistance, compensatory hyperinsulinemia, and increased growth factor production act either independently or synergistically with elevated levels of bioavailable sex hormones to induce some forms of cancer. Adipose tissue is the primary source of endogenous estrogen in postmenopausal women. Estrogen is known to stimulate the production of insulin-like growth factor and amplify its effects on target tissues; the increased mitotic activity provides a fertile environment for carcinogenesis. In line with these observations, the link between obesity and breast cancer appears to be obscured in women who use postmenopausal hormone therapy since the exogenous estrogen effect is greater than the endogenous elevation due to excess weight. In the Nurses’ Health Study, weight gain in adulthood was predictive of incident breast cancer only among women who had never used hormone therapy. Because tumor cells have a greater capacity than noncancerous cells to utilize excess glucose, the proliferation of tumor cells may also be accelerated in obese individuals with glucose intolerance or other metabolic abnormalities. This may be one reason why obese individuals have poorer cancer outcomes than their leaner counterparts; other reasons include greater postoperative complications, greater difficulty on the part of physicians in determining the most effective radiation and chemotherapy doses for heavier patients, and, as mentioned earlier, delayed disease detection.
Non-hormonal pathways linking obesity to other cancers have also been proposed. For example, gastroesophageal reflux disease and impaired esophageal motility are more prevalent among obese individuals and may contribute to the development of esophageal cancer by exposing the mucosal lining of the esophagus to prolonged irritating contact with stomach acids and carcinogens in food.
Although epidemiologic evidence is sparse, intentional weight loss, in part by improving metabolic and endocrine profiles, would be predicted to decrease cancer risk.
The biologic mechanisms by which obesity may raise cancer risk are not entirely clear and may vary by cancer site. It may be that obesity-associated insulin resistance, compensatory hyperinsulinemia, and increased growth factor production act either independently or synergistically with elevated levels of bioavailable sex hormones to induce some forms of cancer. Adipose tissue is the primary source of endogenous estrogen in postmenopausal women. Estrogen is known to stimulate the production of insulin-like growth factor and amplify its effects on target tissues; the increased mitotic activity provides a fertile environment for carcinogenesis. In line with these observations, the link between obesity and breast cancer appears to be obscured in women who use postmenopausal hormone therapy since the exogenous estrogen effect is greater than the endogenous elevation due to excess weight. In the Nurses’ Health Study, weight gain in adulthood was predictive of incident breast cancer only among women who had never used hormone therapy. Because tumor cells have a greater capacity than noncancerous cells to utilize excess glucose, the proliferation of tumor cells may also be accelerated in obese individuals with glucose intolerance or other metabolic abnormalities. This may be one reason why obese individuals have poorer cancer outcomes than their leaner counterparts; other reasons include greater postoperative complications, greater difficulty on the part of physicians in determining the most effective radiation and chemotherapy doses for heavier patients, and, as mentioned earlier, delayed disease detection.
Non-hormonal pathways linking obesity to other cancers have also been proposed. For example, gastroesophageal reflux disease and impaired esophageal motility are more prevalent among obese individuals and may contribute to the development of esophageal cancer by exposing the mucosal lining of the esophagus to prolonged irritating contact with stomach acids and carcinogens in food.
Although epidemiologic evidence is sparse, intentional weight loss, in part by improving metabolic and endocrine profiles, would be predicted to decrease cancer risk.
Liver And Gallbladder Disease
Obesity is often associated with morphological and functional changes in the liver. In one autopsy study, nonalcoholic steatohepatitis, a pathological condition characterized by fatty infiltration, inflammation, and fibrosis, was observed in 18.5% of markedly obese patients and 2.7% of lean patients; severe fibrosis was found in 13.8% of markedly obese patients and 6.6% of lean patients (Wanless and Lentz, 1990). Other series suggest that 70% of patients with nonalcoholic steatohepatitis are overweight. Although usually benign, nonalcoholic steatohepatitis occasionally leads to the development of cirrhosis, portal hypertension, and hepatic failure. The risk of gallbladder disease also rises with increasing weight. Data from NHANES III generally show monotonic relationships between degree of overweight and the prevalence of gallbladder disease among both men and women, with risk ratios jumping from about 1.5 in overweight (BMI 25–29.9) to 2.6 or higher among persons with BMI >40 as compared with those with BMI 18.5 to 24.9 (Must et al., 1999). Prospective cohort studies with long follow-up periods have yielded similar results. In the Harvard Alumni Study, men with BMI 22 to 23.9, 24 to 26.9, and 27 had adjusted relative risks of incident gallbladder disease of 1.5, 1.8, and 2.7, respectively, as compared with men with BMI < 22 (Sahi et al., 1998). In the Nurses’ Health Study, the relative risks of cholecystectomy or new-onset gallstones were 1.7 for women with BMI 24 to 24.9 and 6 for women with BMI≥32, compared with women with BMI < 20. When women with BMI < 25 were used as the referent, the relative risks were 1.9 for women with BMI 25 to 29.9, 2.5 for those with BMI 30 to 34.9, and 3 for those with BMI ≥35. In this cohort, weight cycling was also highly associated with increased risk of cholecystectomy, after adjustment for baseline BMI.Osteoarthritis
Osteoarthritis, a degenerative disease affecting cartilage and bone, is a leading cause of disability and pain among U.S. adults. Osteoarthritis of the knee, hip, and hand accounts for much of this disability among the elderly. Data from NHANES III indicate that the prevalence of osteoarthritis among women rises with increasing body weight, from 52 per 1000 women with BMI 18 to 24.9 to 172 per 1000 for those with BMI 40 (Figure 4). The corresponding figures for men are 26 cases per 1000 individuals in the lower BMI range and 100 cases per 1000 individuals in the upper BMI range.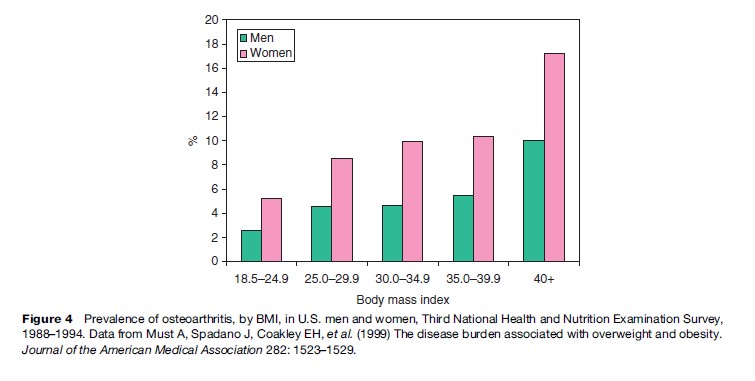 Studies also show an association between excess weight and new-onset osteoarthritis. In the Framingham Study, for example, the risk of developing osteoarthritis of the knee over a 35-year follow-up period was twice as high among obese women and 1.5 times as high among obese men (i.e., those in the heaviest quintile of relative weight) than among their counterparts in the lowest three quintiles of weight. Weight gain was also directly correlated with incident osteoarthritis; the 10-year risk jumped about 40% for each 4.5-kg gain. Similar correlations between obesity and knee osteoarthritis have been observed in other cohort, twin, and national survey studies. Epidemiologic evidence linking obesity and osteoarthritis of the hip or hand is less consistent, however, with some studies showing a positive association and others showing no association. A cross-sectional survey of 809 patients who had undergone knee or hip joint replacement for osteoarthritis found that obesity was strongly associated with bilateral knee osteoarthritis but not with bilateral hip or hand osteoarthritis (Sturmer et al., 2000).
In the Framingham Study, overweight women who lost weight significantly lowered their risk of knee osteoarthritis. For each 2-unit reduction in BMI (about 5 kg for a woman of average height), the risk of osteoarthritis dropped by >50%. It was estimated that if obese women (defined as BMI 29) lost enough weight to fall into the overweight category (BMI 25 to 28.9), and overweight women lost enough weight to fall into the normal-weight category (BMI < 25), the total rate of knee osteoarthritis in the population would drop by one third. Similar estimates for men suggest that such weight reductions would cut the rate of knee osteoarthritis by more than one fifth. Indeed, excess weight accounts for more osteoarthritis than any other known factor among women and is second only to major knee injury as a preventable cause of knee osteoarthritis among men. Among overweight osteoarthritis patients, weight loss achieved through pharmacotherapy, surgery, or diet and exercise has also been associated with an attenuation of clinical symptoms, including pain and functional disability.
Obesity is believed to lead to knee or hip osteoarthritis because of the increased mechanical stress, or force per unit area, on weight-bearing joints. During walking, a force ranging from three to six times one’s body weight is exerted across the knee. Obesity-associated endocrine abnormalities, especially in postmenopausal women, or higher bone mineral densities are other potential pathways by which excess weight could affect the development of not only knee and hip arthritis but also osteoarthritis of the hand, wrist, and other joints.
Studies also show an association between excess weight and new-onset osteoarthritis. In the Framingham Study, for example, the risk of developing osteoarthritis of the knee over a 35-year follow-up period was twice as high among obese women and 1.5 times as high among obese men (i.e., those in the heaviest quintile of relative weight) than among their counterparts in the lowest three quintiles of weight. Weight gain was also directly correlated with incident osteoarthritis; the 10-year risk jumped about 40% for each 4.5-kg gain. Similar correlations between obesity and knee osteoarthritis have been observed in other cohort, twin, and national survey studies. Epidemiologic evidence linking obesity and osteoarthritis of the hip or hand is less consistent, however, with some studies showing a positive association and others showing no association. A cross-sectional survey of 809 patients who had undergone knee or hip joint replacement for osteoarthritis found that obesity was strongly associated with bilateral knee osteoarthritis but not with bilateral hip or hand osteoarthritis (Sturmer et al., 2000).
In the Framingham Study, overweight women who lost weight significantly lowered their risk of knee osteoarthritis. For each 2-unit reduction in BMI (about 5 kg for a woman of average height), the risk of osteoarthritis dropped by >50%. It was estimated that if obese women (defined as BMI 29) lost enough weight to fall into the overweight category (BMI 25 to 28.9), and overweight women lost enough weight to fall into the normal-weight category (BMI < 25), the total rate of knee osteoarthritis in the population would drop by one third. Similar estimates for men suggest that such weight reductions would cut the rate of knee osteoarthritis by more than one fifth. Indeed, excess weight accounts for more osteoarthritis than any other known factor among women and is second only to major knee injury as a preventable cause of knee osteoarthritis among men. Among overweight osteoarthritis patients, weight loss achieved through pharmacotherapy, surgery, or diet and exercise has also been associated with an attenuation of clinical symptoms, including pain and functional disability.
Obesity is believed to lead to knee or hip osteoarthritis because of the increased mechanical stress, or force per unit area, on weight-bearing joints. During walking, a force ranging from three to six times one’s body weight is exerted across the knee. Obesity-associated endocrine abnormalities, especially in postmenopausal women, or higher bone mineral densities are other potential pathways by which excess weight could affect the development of not only knee and hip arthritis but also osteoarthritis of the hand, wrist, and other joints.
